titanium configuration|titanium number of electrons : Clark Titanium is produced commercially by reducing titanium(IV) chloride with magnesium. Titanium(IV) oxide is produced commercially by either the ‘sulfate process’ or the . Characters react to their own series/movies, Reaction fics, spy x family fics read, Any and all reading/watching the future/fanfiction fic, Watching the World Through Other People’s Eyes, Reaction to., Bonkas Read & Watch-It FicsViral Scandal (2021) is a Filipino drama series. A simple, middle-class family is rocked by a scandal when the eldest daughter (Charlie Dizon) is caught.
titanium configuration,Mar 23, 2023 Click on above elements (in Periodic table) to see their information or Visit .Learn how to write the electron configuration of titanium, a transition metal with a gray tint and high corrosion resistance. Find out its physical and chemical properties, common .Titanium is produced commercially by reducing titanium(IV) chloride with magnesium. Titanium(IV) oxide is produced commercially by either the ‘sulfate process’ or the .Titanium is used in steel as an alloying element (ferro-titanium) to reduce grain size and as a deoxidizer, and in stainless steel to reduce carbon content. Titanium is often alloyed with aluminium (to refine grain size), vanadium, copper (to harden), iron, manganese, molybdenum, and other metals. Titanium mill products (sheet, plate, bar, wire, forgings, castings) find application in industrial, aerospace, recreational, and emerging markets. Powdered titanium is used in pyrotechnics
Titanium Electron Configuration: Titanium is a chemical element that has a chemical symbol Ti. Its atomic number is 22. It is a transition metal lustrous which has a silver colour, low density, and .
Electron Configuration for Ti , Ti3+, and Ti4+ (Titanium and Titanium Ions)Titanium electron configuration. ← Electronic configurations of elements. Ti (Titanium) is an element with position number 22 in the periodic table. Located in the IV period. .
Titanium is the 22nd element of the periodic table and its atomic number is 22, which means it has 22 electrons in the ground state. There are three types of .In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic . To write the configuration for the Titanium ions, first we need to write the electron configuration for just Titanium (Ti). We first need to find the number.

Ti I Ground State 1s 2 2s 2 2p 6 3s 2 3p 6 3d 2 4s 2 3 F 2 Ionization energy 55072.5 cm-1 (6.82812 eV) Ref. SZK90 Ti II Ground State 1s 2 2s 2 2p 6 3s 2 3p 6 3d 2 4s 4 F 3 / 2 Ionization energy 109494 cm-1 (13.5755 eV) Ref. SC85-1 (13.5755 eV) Ref. SC85
Chemistry of Titanium is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. Discovered independently by William Gregor and Martin Klaproth in 1795, titanium (named for the mythological Greek Titans) was first isolated in 1910. Gregor, a Cornish vicar and amateur ..Electron Configuration for Ti , Ti3+, and Ti4+ (Titanium and Titanium Ions)
This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.. But wait — you can avoid .
Version (Includes PCIE 4.0 Riser) Version 2.1 Variant restocking or unavailable Side Panels Aluminum Coated (Black Color) Variant restocking or unavailable CNC Anodized (Black Color) Variant restocking or unavailable CNC Anodized (Titanium Color) Variant restocking or unavailable Ships Within 3-7 days (International) Variant restocking or unavailable
Introduction to electron configurations. Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of .
For the transition metals, groups 3-12, there are many exceptions. The general rule is that the element's electron configuration ends in d and whatever place they are in. Scandium would end in 3d1, titanium in 3d2, etc. The transition metals are behind by one period because the d electrons are high in energy. This video shows how to draw the orbital diagram of Titanium (Ti). It also shows how to write the electron configuration of titanium and the shorthand noble.Electron Configuration [Ar] 3d 2 4s 2. Ti. Titanium is one of the few elements that burns in pure nitrogen gas. Physical Properties. Phase. Solid. Density. 4.54 g/cm 3. . Titanium has potential use in .titanium number of electrons In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x. The energy level, "n", can be determined based on the periodic table, simply by looking at the row number in which the element is in. However, there is an exception for the d-block and f-block, in which the energy level, "n" for the d block is .In several cases, the ground state electron configurations are different from those predicted by Figure 6.8.1 6.8. 1. Some of these anomalies occur as the 3 d orbitals are filled. For example, the observed ground state electron configuration of chromium is [Ar]4 s1 3 d5 rather than the predicted [Ar]4 s2 3 d4.

Properties. Titanium has a melting point of 1660 +/- 10°C, boiling point of 3287°C, specific gravity of 4.54, with a valence of 2, 3, or 4. Pure titanium is a lustrous white metal with low density, high strength, and high corrosion resistance. It is resistant to dilute sulfuric and hydrochloric acids, moist chlorine gas, most organic acids .The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = + 1 2 ).
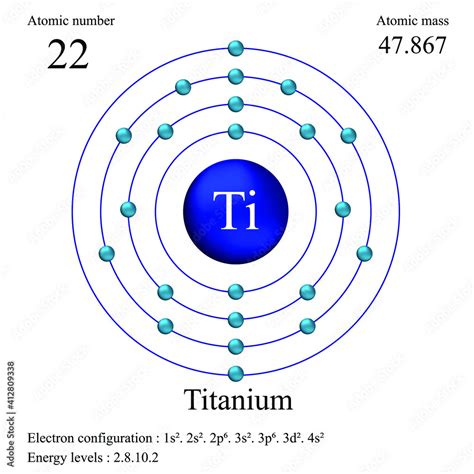
titanium configurationElectron configuration of Titanium is [Ar] 3d2 4s2. Possible oxidation states are +2,3,4. The +4 oxidation state dominates titanium chemistry, but compounds in the +3 oxidation state are also common. Because of its high oxidation state, titanium (IV) compounds exhibit a high degree of covalent bonding.Titanium. Full electron configuration of titanium: 1s2 2s2 2p6 3s2 3p6 3d2 4s2. scandium ← titanium → vanadium.titanium configuration titanium number of electronsElectron configuration of the titanium(II) ion Ti 2+ is [Ar] 3d 2 Titanium(II) chloride TiCl 2 is a black solid. The octahedral violet hexaaquatitanium(II) ion [Ti(H 2 O) 6 )] 2+ ion can be formed by reducing Ti(IV) or Ti(III) with a metal/acid mixture but it is very unstable in redox terms, ie readily oxidised by dissolved oxygen from the .
titanium configuration|titanium number of electrons
PH0 · titanium number of electrons
PH1 · noble gas configuration titanium
PH2 · ground state electron configuration titanium
PH3 · Iba pa